Abstract
Background: The incidence and severity of acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic stem cell transplantation (alloHSCT) is decreased with T-cell depletion. However, ~14% of these T-cell depleted (TCD) recipients will have aGVHD with skin involvement (Barba et al. 2017). Furthermore, alloHSCT recipients are at increased risk for drug eruptions and infectious exanthems (Byun et al. 2011). While histopathologic differences have been reported between aGVHD and non-aGVHD rash in TCD recipients (Fischer et al. 2015), skin biopsies alone are insufficient to determine rash etiology. As such, distinguishing inflammatory non-aGVHD rashes from aGVHD of the skin after TCD HSCT remains challenging and relies on clinical presentation. While peripheral eosinophilia is seen in both aGVHD and drug hypersensitivity, rashes that present with concomitant eosinophilia after HSCT are often suspected to have a drug-induced etiology. We sought to assess the incidence and features of aGVHD and non-aGVHD rashes within 1 year after TCD alloHSCT, as well as common etiologies of non-aGVHD rash. These findings may guide clinicians in earlier diagnosis and management of non-aGVHD rash.
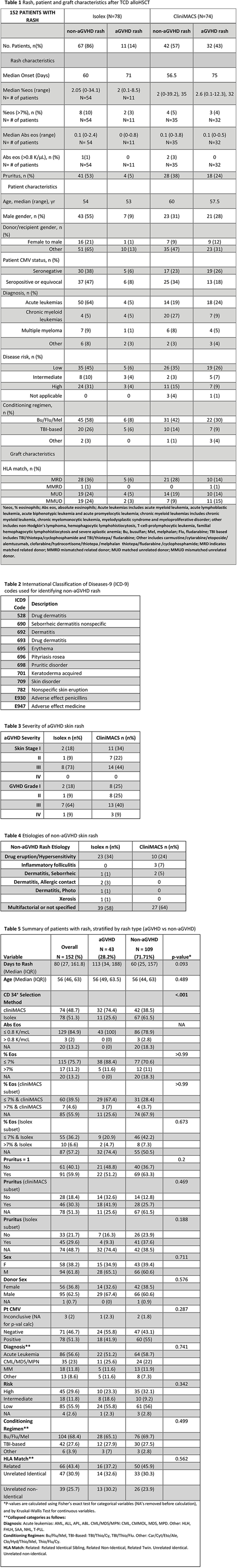
Methods: Using a clinical research database, 243 adult patients were identified who received alloTCD peripheral blood stem cell transplantation (PBSCT) at a single institution between 2008 and 2011. All patients had CD34+ hematopoietic progenitor cells selected using: the Isolex 300i Magnetic Cell, followed by additional T-cell rosetting with neuraminidase-treated sheep erythrocytes or using the CliniMACS CD34+ Reagent System (Table 1). Given decreased incidence of aGVHD with Isolex versus CliniMACS (Barba et al. 2017), we established Isolex and CliniMACS TCD groups and assessed aGVHD and non-aGVHD rash within these two CD34+ selection modalities. To identify non-aGVHD patients with skin rash, charts were reviewed from date of HSCT through 1 year post HSCT by review of dermatology visit notes or by extraction of International Classification of Diseases-9 (ICD-9) codes for skin lesion or rash (Table 2); skin infections and chronic GVHD rashes were excluded. Acute GVHD was diagnosed with histological confirmation when clinically indicated. Rash characteristics, including pruritus and peripheral eosinophilia at onset of rash, were collected from charts of both non-aGVHD and aGVHD rash patients.
Results: Among 243 TCD PBSCT transplant recipients, 152 patients (63%) were identified with skin rash within 1 year after HSCT. Of these patients, 43 had aGVHD rash and 109 had non-aGVHD rash. The majority of aGVHD rashes had skin stage III aGVHD regardless of CD34+ selection method (Table 3). For patients with non-aGVHD rash, etiologies included inflammatory conditions (Table 4). TCD by Isolex led to non-aGVHD rash development at a median onset of 60 days and aGVHD rash at a median onset of 71 days; while TCD by CliniMACS led to non-aGVHD rash development at a median onset of 56.5 days and aGVHD rash at a median onset of 75 days. Of patients who had recorded complete blood counts at onset of rash, elevated percent eosinophilia (% eosinophils >7%) was present in 10% of Isolex and 5% of CliniMACS non-aGVHD rashes versus 3% of Isolex and 4% of CliniMACS aGVHD rashes. Peripheral eosinophilia was not associated with aGVHD versus non-aGVHD skin rash post TCD HSCT (p≥0.99) nor when separated by CD34+ selection method (Isolex p=0.673; CliniMACS p≥0.99). While non-aGVHD skin rash patients had higher incidence of pruritus compared to aGVHD skin rash, pruritus was not a significant predictor of aGVHD versus non-aGVHD skin rash (p=0.20) nor when separated by CD34+ selection modality (Isolex p=0.188; CliniMACS p=0.469).
Conclusions: In our case series of 243 TCD PBSCT recipients of whom 152 had skin rashes, over three-quarters of all non-aGVHD skin rashes with clear etiologies were attributed to drug eruptions. Our results suggest that the commonly utilized feature of peripheral eosinophilia may not be helpful in differentiating between aGVHD and drug rashes after TCD alloHSCT. Additionally, pruritus at rash onset was not helpful in distinguishing cause of rash as due to aGVHD or non-aGVHD after TCD alloHSCT. Based on these data, if clinical scenario supports GVHD associated rash, the presence of peripheral eosinophilia or pruritus should not delay initiation of therapy for GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal